info@universallab.org
WhatsApp: +41762172997


Price may vary based on selected options
Delivery time: 1 ~ 2 weeks

X-Ray Photoelectron Spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition, chemical state, and electronic state of the elements within a material. It is particularly useful for analyzing the surface chemistry of a wide range of materials.
The following table provides a comparative overview of several commonly used techniques for elemental composition and chemical state analysis, including XPS, AES, SIMS, and EDS/EDX. The table summarizes the main information each technique can provide, analysis depth, sensitivity, quantitative capability, typical application scenarios, and spatial resolution.
XPS spectra generally include photoelectron peaks, satellite peaks, Auger electron peaks, and spin-orbit splitting (SOS), among others.

It is characterized by a small analysis area, shallow analysis depth, and non-destructive nature. XPS is widely applied in the study of various materials, including metals, inorganic materials, catalysts, polymers, coating materials, and minerals, as well as in the study of processes such as corrosion, friction, lubrication, adhesion, catalysis, coating, and oxidation.
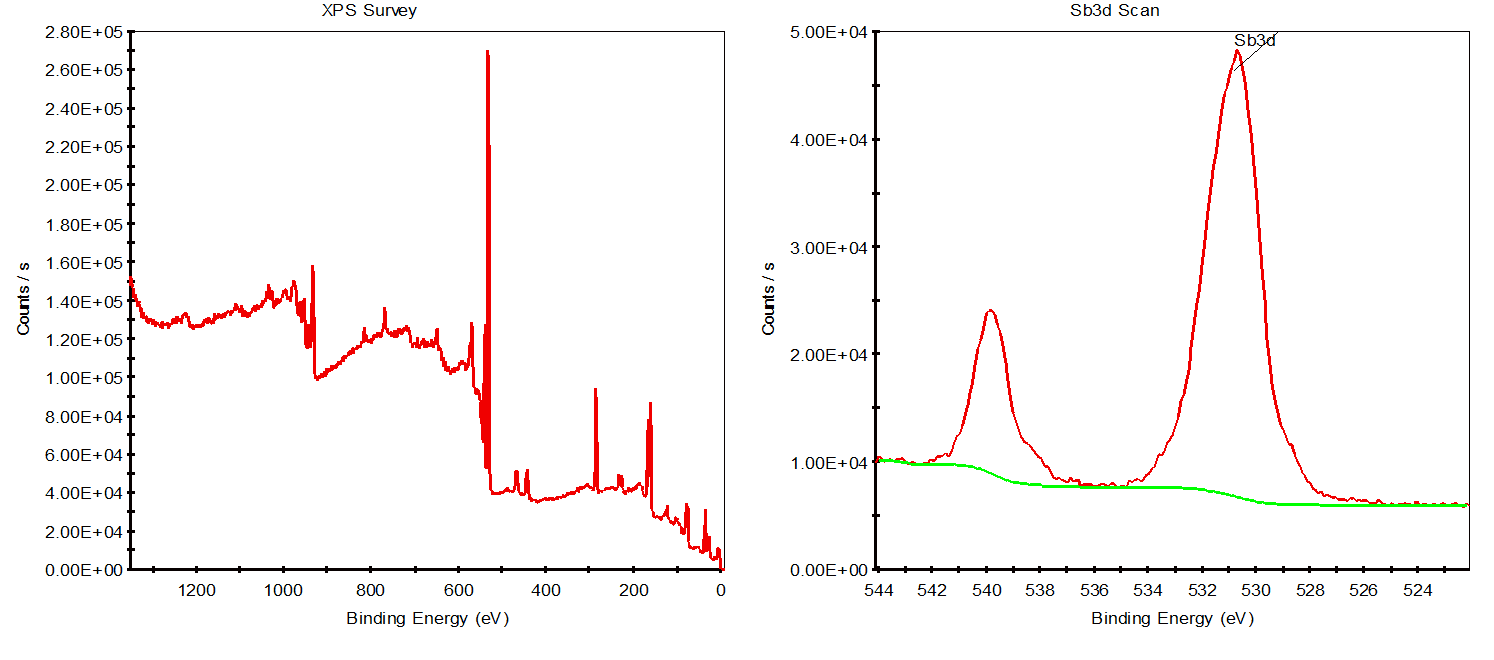
The survey spectrum on the left displays the binding energy distribution of all elements present on the sample surface. By analyzing the positions and intensities of these peaks, the elemental composition of the sample can be preliminarily determined, such as the presence of Sb, S, C, O, and other elements.
The right panel shows a high-resolution scan of the antimony (Sb) 3d region. The two main peaks correspond to the Sb 3d5/2 and Sb 3d3/2 spin-orbit components, which are characteristic features of antimony. The positions and shapes of these peaks can be used to analyze the chemical state of antimony (e.g., Sb³⁺ or Sb⁵⁺) as well as its content in the sample. The green curve represents the background subtraction, while the red curve is the actual signal.
XPS analysis is widely used for characterizing the surface elemental composition and chemical states of nanomaterials, organometallic complexes, thin films, and catalysts. It is commonly employed to verify the successful incorporation of target elements, changes in oxidation states, and the effectiveness of surface modifications.
X-ray Photoelectron Spectroscopy (XPS) is a powerful and widely used technique for surface analysis, providing detailed information about the elemental composition, chemical states, and electronic structure of materials within the top few nanometers. Its high surface sensitivity and ability to distinguish chemical states make it invaluable in fields such as materials science, surface chemistry, catalysis, and semiconductor research.
| Technique | Main Information Provided | Detection Depth | Lateral Resolution | Sensitivity | Quantitative Analysis | Typical Applications | Limitations |
|---|---|---|---|---|---|---|---|
| XPS | Elemental composition, chemical state | 1–10 nm (surface) | ~10–100 μm | 0.1–1 at% | Yes | Surface chemistry, thin films, polymers | Requires UHV, limited to surface, slow scan |
| Technique | Main Information Provided | Detection Depth | Lateral Resolution | Sensitivity | Quantitative Analysis | Typical Applications | Limitations |
|---|---|---|---|---|---|---|---|
| XPS | Elemental composition, chemical state | 1–10 nm (surface) | ~10–100 μm | 0.1–1 at% | Yes | Surface chemistry, thin films, polymers | Requires UHV, limited to surface, slow scan |
| AES | Elemental composition, chemical state | 1–5 nm (surface) | ~10 nm | 0.1–1 at% | Yes | Thin films, micro-area analysis | Requires UHV, less sensitive to light elements |
| SIMS | Elemental/isotopic composition, depth profile | less than1 nm (surface), depth profiling | ~50 nm–1 μm | ppm–ppb | Semi-quantitative | Trace analysis, depth profiling | Matrix effects, complex quantification |
| EDS/EDX | Elemental composition | ~1 μm (bulk/surface) | ~1 μm | ~0.1 wt% | Semi-quantitative | Bulk analysis, inclusions, mapping | Poor for light elements, lower surface sensitivity |
No, it is not. The sensitivity factor of the main peak is different for each element.
You should look at the magnitude of the residuals—the smaller, the better. You also need to consider the physical meaning of the fit. The number of peaks to fit depends on the actual situation of the sample and the degree of fit; there is no strict rule as to which is better.
XPS (X-Ray Photoelectron Spectroscopy) is a technique that measures the energy distribution of photoelectrons emitted from a sample surface under X-ray irradiation. It enables qualitative and semi-quantitative analysis of surface elemental composition, chemical states, and molecular structure. XPS is widely used in materials science, surface chemistry, and semiconductor research for its high surface sensitivity and ability to provide detailed chemical information.